Tamily Weissman-Unni and the Amazing Technicolor Brain
Open gallery

“Beauty is truth, truth beauty,”—that is all
Ye know on earth, and all ye need to know.
—John Keats
By Geoff Koch
Photos by Steve Hambuchen
Say you want to understand the flow of goods and people between Portland and Seattle. Sure, you’ll need to start with good maps of the roads. But eventually you’ll need lots more. One hypothetical example: What can you say about those two tractor-trailers that travel north on I-5 every Tuesday morning? Are they caravanning to the same destination, or do their schedules just happen to coincide? To really understand the network well enough to explain why the traffic snarls one moment and flows smoothly the next, you’ll need a diagram that somehow represents the relationship among the individual vehicles on the roads and how these relationships change over time.
This is, roughly speaking, the same reason Tamily Weissman-Unni makes diagrams and maps of neural circuitry within the humble 1-inch-long zebrafish, whose small brains she lights up like the brilliant rainbows that occasionally appear in the skies above Portland when the sun peeks through the rain. More specifically, she wants to understand the neural network, down to the level of individual neurons—the cells in the brain that transmit information much like transistors do in computer chips. Her pictures of these technicolor brains, which show individual nerve cells aglow in a spectrum of fluorescent colors, can help explain how neural connections form in zebrafish—uncovering principles likely to be directly relevant to the human brain.
Her work is part of larger effort in labs around the world to ultimately build a detailed wiring diagram, or connectome, of the human brain. Because of technical challenges associated with making sense of the 100 billion or so neurons tangled like spaghetti in your head, the connectome won’t be finished for at least decades, or maybe not ever. This hasn’t stopped an array of predictions about what such a map might be good for, predictions that are either exhilarating or alarming, depending on your point of view. Search for “transhumanism+connectome” online and you’ll soon stumble on breathless anticipation of the sci-fi-like moment when it first will be possible—this century, technophiles insist—to upload the contents of a human brain to a computer.
More Than a Pretty Picture
Sitting in her second-floor office in the Bio-Psych building, Weissman-Unni smiles and rolls her eyes in good-natured exasperation when the uploading idea is mentioned. The assistant professor of biology, in her second year at Lewis & Clark after several years of research and teaching at Harvard, has obviously heard such hype before.
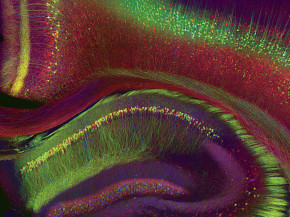
Weissman-Unni’s office is decorated with images from her past and present research, several of which have appeared in the world’s most prominent scientific journals, including Nature. (She publishes under the name Weissman.) The pictures are visually striking, an opinion that’s apparently shared by many. Weissman-Unni’s images have won photo contests, been used as cover images for books and magazines, been featured in traveling art exhibits in Europe, and hung in living rooms.
“The biggest surprise is just how unbelievably beautiful it is,” says Josh Sanes, a professor of molecular and cellular biology at Harvard who collaborated with Weissman-Unni. Sanes is speaking of pictures of a multihued mouse cerebellum lit up like a Christmas tree, among those on display in Weissman-Unni’s office.
The Harvard team, which also included Jean Livet and Jeff Lichtman, had worked for years with only limited success on the genetic manipulation that caused the neurons to glow. Their building blocks: genes strung together from jellyfish and coral with instructions for building colored proteins; a system for scrambling these genes like a blackjack dealer shuffles a deck of cards; and lots of trial and error inserting the genes and the shuffling mechanism into the brains of mice and watching for the lights to turn on.
“I remember the day I was in the microscope room and took that image of the mouse cerebellum,” says Weissman-Unni, gesturing to a picture on her wall. “It was unbelievable. I mean, I had never seen anything like it. I ran to get Jean [Livet] and Jeff [Lichtman].”
One surprise was that the neurons were lit up in an array of “brainbow” colors, not just in the expected red, green, and blue. It turns out that there were several copies of the genes for each color. So the shuffling mechanism produced a random blend of the colors that differed with each cell. In one neuron, blue and red combined to make purple. In another, red and yellow combined to make orange. And the images, combined with the rigorous methodology behind them, made one of the biggest neuroscience stories of the decade.

Publicity, either among peers or in the world at large, has little to do with personal fulfillment. None other than Elvis Presley said, “The image is one thing and the human being is another… . It’s very hard to live up to an image, put it that way.” Weissman-Unni, who herself once toyed with trying to make it as a singer—“my voice wasn’t big enough”—clearly enjoyed doing the research. However, her passion was pointing her in a direction that combines her research with teaching.
Weissman-Unni wound up as a head teaching fellow and assistant director for Harvard’s undergraduate neurobiology concentration, which grew into the largest such biology concentration under her watch. She moved to Lewis & Clark in 2011, excited to become an assistant professor and begin her own independent research program. Her hope in moving, which has come true so far, is that in Portland she would be able to continue to work with students and do research, often at the same time.
“I’m so impressed with the students here; they truly wish to be challenged and go in-depth, not just earn a grade,” says Weissman-Unni. “They are easily as smart and talented as students I worked with at Harvard.”
First Playing Cupid, Then Painting Pisces in an Array of Pastels
Zac Tobias was one of these students. At the start of his senior year, in fall 2011, he took Weissman-Unni’s 400-level whimsically named Neuroanatomically Correct class. It was the first neuroscience course for the San Diego native, a biology major who’d also studied Spanish and music and had never heard of a zebrafish, which Weissman-Unni uses as the basis of her brainbow research at Lewis & Clark. Now he’s, well, intimately familiar with the fish.
“In the wild, zebrafish mate at dawn,” says Tobias, who graduated with a biology degree in May 2012 and has worked full time managing the Weissman-Unni lab ever since. Tobias opens the door to a small room off the main lab that houses racks of aquarium tanks full of darting zebrafish. The real action apparently takes place on an opposite countertop, where several shoe-box-size plastic containers are visible, each full to the brim with water and a dozen or so fish. A closer look reveals that each of these smaller tanks is divided in two with a clear plastic window, with roughly equal numbers of fish on either side.

Tobias collects the fertilized eggs, then injects them with a small bit of the red-yellow-blue series of brainbow genes. A few days later—it takes just 48 hours for the eggs to hatch and the small larvae to start swimming and feeding—Tobias is using a sophisticated microscope at Oregon Health & Science University to take pictures of fish painted in kaleidoscopic colors. (Weissman-Unni is working to obtain funds to bring one of these microscopes to the Lewis & Clark biology department.)
As was true in the mouse, the brainbow effect makes it easy to distinguish individual cells in the zebrafish brain. Weissman-Unni and Tobias hope to take advantage of these clear demarcations to better understand a process: how neurons develop into complex circuits, or networks. An open question is how pruning of networks may be associated with learning and development in the brain.
Scientists already understand how synaptic pruning takes place where neurons meet muscle fibers. Babies and toddlers can struggle to grasp a block or a toy in part because the muscle fibers in their arms and hands have too many inputs from neurons. Think here of trying to operate a remote control airplane holding a dozen controllers when just one would do. By the time children are two years old, grasping is easy, in part because the number of neurons that input to each muscle fiber is reduced to one. This developmental pattern of pruning, in essence trial and error, may be a general approach that the nervous system uses to solve the daunting puzzle of how to create highly specific yet complex networks that precisely control behavior.
Seeing synapses wither away is easy enough when you’re looking at connections between two different types of cells, neurons and muscle cells, usually connected in a one-to-one fashion. But what about when you’re trying to distinguish connections among hundreds of cells of similar type? After all, the brain is mostly loaded with neurons, albeit arrayed in impossibly interconnected circuits. One neuron in the cerebral cortex, the seat of thought, language, and consciousness, can have thousands of inputs.
Weissman-Unni focuses on the cerebellum, which handles motor control, in part because its web of connections is only moderately complex. Think of mapping the network of roads in Sioux Falls, South Dakota, versus doing the same in Los Angeles. The general principles that underlie that Dakotan road map, however, should be similar to those in L.A. This middling complexity, where synaptic junctions are measured by the tens instead of the thousands, is perfect for the brainbow technique, which can generate at least 100 different colors.
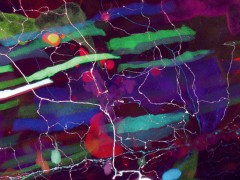
The lab is sprinkled with pictures of fluorescent Haight-Ashbury fish that are at once striking and, unfortunately, somewhat difficult to study. The reason: the bright colors are expressed throughout the fish’s body and tend to drown out the lit-up cells in the cerebellum that Weissman-Unni is most interested in.
Turn Off the Lights so We Can See
Senior biology major Ian Lake is trying to remedy matters by making tweaks to the bit of DNA inserted into the eggs. In essence, he’s trying to insert a locked door in front of the brainbow genes that could be opened by a specific key found only in specific neurons. His goal, elusive for now though fun to pursue, is to turn off most of the lights so that the particular network in the cerebellum blazes more brightly.
“Tamily has set up a really nice environment in the lab, where you have a purpose but are not stressed out about actually achieving that in as little time as possible,” says Lake, who is applying to graduate programs in education and hopes to someday work as a high school science teacher. “She encourages the enjoyment of the process and appreciation of something that’s really beautiful to look at. You can actually look at a living zebrafish under a microscope and see its brain cells fluorescing in a rainbow of colors. In the background, you can see its heart beating and its blood flowing.”
Tamily Weissman-Unni’s current research is generously supported by the M.J. Murdock Charitable Trust in Vancouver, Washington.
Geoff Koch is a science and technology writer in Portland.
More L&C Magazine Stories
Lewis & Clark Magazine is located in McAfee on the Undergraduate Campus.
MSC: 19
email magazine@lclark.edu
voice 503-768-7970
fax 503-768-7969
The L&C Magazine staff welcomes letters and emails from readers about topics covered in the magazine. Correspondence must include your name and location and may be edited.
Lewis & Clark Magazine
Lewis & Clark
615 S. Palatine Hill Road MSC 19
Portland OR 97219

